Sulfur dioxide and oxygen are two gases that play a crucial role in various industrial and environmental processes. One of the most significant reactions involving these two gases is the formation of sulfur trioxide. In this article, we will delve into the world of sulfur dioxide and oxygen, exploring their properties, the reaction that forms sulfur trioxide, and the significance of this reaction in various industries.
Understanding Sulfur Dioxide and Oxygen
Sulfur dioxide (SO2) is a colorless gas with a pungent, irritating odor. It is a common air pollutant, primarily produced by the burning of fossil fuels, such as coal and oil, and the smelting of sulfur-containing ores. SO2 is also a key component in the production of sulfuric acid, which is used in various industrial processes.
Oxygen (O2), on the other hand, is a vital component of the Earth's atmosphere, making up approximately 21% of the air we breathe. Oxygen is essential for the survival of most living organisms and is also used in various industrial processes, such as steel production and wastewater treatment.

The Reaction: Sulfur Dioxide and Oxygen Form Sulfur Trioxide
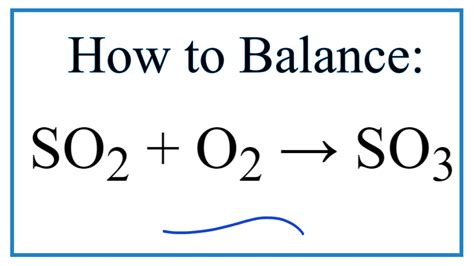
The reaction between sulfur dioxide and oxygen to form sulfur trioxide is a reversible reaction, meaning it can proceed in both the forward and reverse directions. The reaction is as follows:
2SO2 + O2 ⇌ 2SO3
In this reaction, two molecules of sulfur dioxide react with one molecule of oxygen to form two molecules of sulfur trioxide. The reaction is exothermic, meaning it releases heat energy, and is often used in the production of sulfuric acid.
Mechanism of the Reaction
The reaction between sulfur dioxide and oxygen to form sulfur trioxide involves a complex mechanism, which can be summarized as follows:
- Initiation: Sulfur dioxide molecules (SO2) collide with oxygen molecules (O2), forming a reactive intermediate.
- Propagation: The reactive intermediate reacts with another sulfur dioxide molecule, forming sulfur trioxide (SO3).
- Termination: The sulfur trioxide molecule reacts with another oxygen molecule, forming a stable product.
This mechanism is catalyzed by vanadium pentoxide (V2O5), which is commonly used in the production of sulfuric acid.
Industrial Significance of the Reaction
The reaction between sulfur dioxide and oxygen to form sulfur trioxide is crucial in various industrial processes, including:
- Sulfuric Acid Production: Sulfur trioxide is used to produce sulfuric acid, which is a key component in the production of fertilizers, pesticides, and pharmaceuticals.
- Steel Production: Sulfur trioxide is used as a flux in the production of steel, helping to remove impurities and improve the quality of the steel.
- Wastewater Treatment: Sulfur dioxide is used to remove impurities and improve the quality of wastewater.
Environmental Impact of the Reaction
The reaction between sulfur dioxide and oxygen to form sulfur trioxide has significant environmental implications. Sulfur dioxide is a major air pollutant, contributing to acid rain and respiratory problems. The reaction can also lead to the formation of ground-level ozone, which can exacerbate respiratory problems.
Practical Applications of the Reaction
The reaction between sulfur dioxide and oxygen to form sulfur trioxide has various practical applications, including:
- Sulfuric Acid Production: Sulfuric acid is used in various industries, including fertilizers, pesticides, and pharmaceuticals.
- Steel Production: Sulfur trioxide is used as a flux in the production of steel, improving the quality of the steel.
- Wastewater Treatment: Sulfur dioxide is used to remove impurities and improve the quality of wastewater.

Factors Affecting the Reaction
Several factors can affect the reaction between sulfur dioxide and oxygen to form sulfur trioxide, including:
- Temperature: The reaction is exothermic, meaning it releases heat energy. Increasing the temperature can increase the rate of reaction.
- Pressure: Increasing the pressure can increase the rate of reaction.
- Catalyst: Vanadium pentoxide (V2O5) is commonly used as a catalyst to improve the rate of reaction.
Conclusion: The Importance of Understanding the Reaction
The reaction between sulfur dioxide and oxygen to form sulfur trioxide is a complex process with significant industrial and environmental implications. Understanding this reaction is crucial for improving the efficiency and safety of various industrial processes, as well as mitigating the environmental impacts of sulfur dioxide emissions. By recognizing the importance of this reaction, we can develop more effective strategies for managing sulfur dioxide emissions and improving the quality of our environment.
We hope this article has provided you with a comprehensive understanding of the reaction between sulfur dioxide and oxygen to form sulfur trioxide. If you have any questions or comments, please feel free to share them with us.
What is the significance of the reaction between sulfur dioxide and oxygen to form sulfur trioxide?
+The reaction between sulfur dioxide and oxygen to form sulfur trioxide is crucial in various industrial processes, including sulfuric acid production, steel production, and wastewater treatment.
What are the environmental implications of the reaction between sulfur dioxide and oxygen to form sulfur trioxide?
+The reaction can lead to the formation of ground-level ozone, which can exacerbate respiratory problems. Sulfur dioxide is also a major air pollutant, contributing to acid rain and respiratory problems.
What are the practical applications of the reaction between sulfur dioxide and oxygen to form sulfur trioxide?
+The reaction has various practical applications, including sulfuric acid production, steel production, and wastewater treatment.