The world of chemistry is fascinating, and understanding the building blocks of matter is crucial for grasping various concepts. One such concept is the electron configuration of elements, which plays a vital role in determining their chemical properties. In this article, we'll delve into the sodium electron configuration, exploring its importance, structure, and relevance in the world of chemistry.

Understanding Electron Configuration
Before diving into the sodium electron configuration, it's essential to understand the basics of electron configuration. Electron configuration is the arrangement of electrons in an atom, which is described using a specific notation. This notation takes into account the energy levels, subshells, and orbitals that electrons occupy. The electron configuration of an element determines its chemical properties, such as reactivity, electronegativity, and ionization energy.
Sodium Electron Configuration
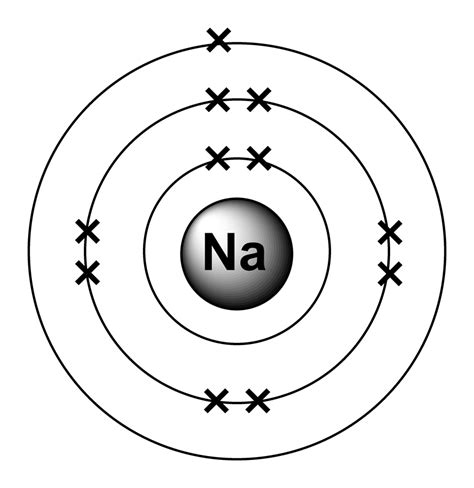
Sodium is an alkali metal with the atomic number 11, which means it has 11 electrons. The electron configuration of sodium is:
1s² 2s² 2p⁶ 3s¹
Breaking down this notation, we can see that:
- The first energy level (1s) is fully occupied with two electrons.
- The second energy level (2s) is fully occupied with two electrons.
- The second energy level (2p) is fully occupied with six electrons.
- The third energy level (3s) has one electron.
This electron configuration indicates that sodium has a single electron in its outermost energy level, which makes it highly reactive. The single electron in the 3s orbital is easily lost, resulting in the formation of a positive ion (Na⁺).
Importance of Sodium Electron Configuration
The sodium electron configuration is crucial in understanding the chemical properties of sodium. Some of the key importance of sodium electron configuration includes:
- Reactivity: The single electron in the 3s orbital makes sodium highly reactive, as it can easily lose this electron to form a positive ion.
- Electronegativity: Sodium has a low electronegativity value, which means it can easily lose electrons to form bonds with other elements.
- Ionization Energy: The ionization energy of sodium is relatively low, which means it can easily lose its outermost electron to form a positive ion.

Applications of Sodium Electron Configuration
The sodium electron configuration has numerous applications in various fields, including:
- Chemical Reactions: Understanding the sodium electron configuration is essential in predicting the outcome of chemical reactions involving sodium.
- Batteries: Sodium is used in batteries, such as sodium-ion batteries, which rely on the electron configuration of sodium to store energy.
- Pharmaceuticals: Sodium is used in various pharmaceutical applications, such as sodium-based medications, which rely on the electron configuration of sodium to interact with biological molecules.
Chemical Reactions Involving Sodium
Sodium is highly reactive, and its electron configuration plays a crucial role in determining the outcome of chemical reactions. Some common chemical reactions involving sodium include:
- Reaction with Water: Sodium reacts with water to form sodium hydroxide and hydrogen gas.
- Reaction with Chlorine: Sodium reacts with chlorine to form sodium chloride (table salt).
- Reaction with Oxygen: Sodium reacts with oxygen to form sodium oxide.

Comparison with Other Elements
The sodium electron configuration is unique compared to other elements. Some key differences include:
- Lithium: Lithium has a similar electron configuration to sodium, but with a smaller atomic number.
- Potassium: Potassium has a similar electron configuration to sodium, but with a larger atomic number.
- Calcium: Calcium has a different electron configuration than sodium, with a full outer energy level.

Conclusion and Future Directions
In conclusion, the sodium electron configuration is a fundamental concept in chemistry that determines the chemical properties of sodium. Understanding the sodium electron configuration is essential in predicting the outcome of chemical reactions and designing new materials and applications. As research continues to advance, we can expect to see new applications of sodium electron configuration in various fields, such as energy storage and pharmaceuticals.
We hope this article has provided you with a comprehensive understanding of the sodium electron configuration. If you have any questions or comments, please feel free to share them below.
What is the electron configuration of sodium?
+The electron configuration of sodium is 1s² 2s² 2p⁶ 3s¹.
Why is sodium highly reactive?
+Sodium is highly reactive due to its single electron in the 3s orbital, which can easily be lost to form a positive ion.
What are some common applications of sodium electron configuration?
+Sodium electron configuration has applications in chemical reactions, batteries, and pharmaceuticals.