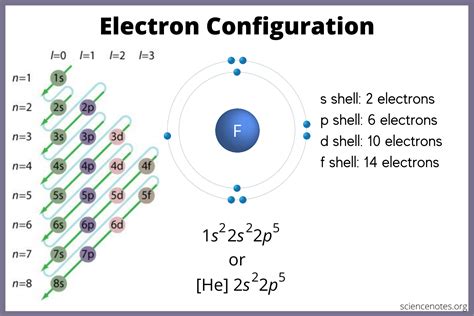
Understanding the electron configuration of an atom is crucial in chemistry, as it helps us predict the properties and behavior of elements. However, writing the electron configuration in the long form can be a daunting task, especially for those who are new to chemistry. In this article, we will break down the process of writing electron configurations in the long form and provide tips and examples to make it easier.
What is Electron Configuration?
Electron configuration is a way of describing the arrangement of electrons in an atom. It is a crucial concept in chemistry, as it helps us understand the properties and behavior of elements. The electron configuration is written in a specific format, with the energy level, orbital type, and number of electrons in each orbital.
The Aufbau Principle and the Periodic Table
The Aufbau principle states that electrons fill the lowest available energy levels in an atom. This principle is used to determine the electron configuration of an atom. The periodic table is arranged in a way that elements with similar electron configurations are placed in the same group or period.

Writing Electron Configuration in the Long Form
Writing electron configuration in the long form involves listing the energy level, orbital type, and number of electrons in each orbital. The format is as follows:
1s² 2s² 2p⁶ 3s² 3p⁶ 4s² 3d¹⁰ 4p⁶
Here's a breakdown of what each part means:
- 1s²: The energy level is 1, the orbital type is s, and there are 2 electrons in this orbital.
- 2s²: The energy level is 2, the orbital type is s, and there are 2 electrons in this orbital.
- 2p⁶: The energy level is 2, the orbital type is p, and there are 6 electrons in this orbital.
- 3s²: The energy level is 3, the orbital type is s, and there are 2 electrons in this orbital.
- 3p⁶: The energy level is 3, the orbital type is p, and there are 6 electrons in this orbital.
- 4s²: The energy level is 4, the orbital type is s, and there are 2 electrons in this orbital.
- 3d¹⁰: The energy level is 3, the orbital type is d, and there are 10 electrons in this orbital.
- 4p⁶: The energy level is 4, the orbital type is p, and there are 6 electrons in this orbital.
Steps to Write Electron Configuration in the Long Form
Here are the steps to write electron configuration in the long form:
- Determine the number of electrons: Find the number of electrons in the atom by looking at the atomic number on the periodic table.
- Determine the energy levels: Determine the number of energy levels in the atom by looking at the periodic table. The energy levels are represented by the rows on the periodic table.
- Determine the orbital type: Determine the orbital type (s, p, d, or f) for each energy level.
- Determine the number of electrons in each orbital: Use the Aufbau principle to determine the number of electrons in each orbital.
Examples of Electron Configuration in the Long Form
Here are some examples of electron configuration in the long form:
- Hydrogen: 1s¹
- Helium: 1s²
- Oxygen: 1s² 2s² 2p⁶ 3s² 3p⁴
- Nitrogen: 1s² 2s² 2p⁶ 3s² 3p³
Tips and Tricks
Here are some tips and tricks to make writing electron configuration in the long form easier:
- Use the periodic table: The periodic table is a valuable resource when writing electron configuration. Use it to determine the number of electrons, energy levels, and orbital type.
- Use the Aufbau principle: The Aufbau principle is used to determine the number of electrons in each orbital. Use it to ensure that the electrons are filled in the correct order.
- Check your work: Double-check your work to ensure that the electron configuration is correct.
Common Mistakes
Here are some common mistakes to avoid when writing electron configuration in the long form:
- Incorrect number of electrons: Make sure to include the correct number of electrons in the electron configuration.
- Incorrect orbital type: Make sure to include the correct orbital type (s, p, d, or f) for each energy level.
- Incorrect number of electrons in each orbital: Make sure to include the correct number of electrons in each orbital.

Conclusion
Writing electron configuration in the long form can be a challenging task, but with practice and patience, it becomes easier. By following the steps outlined in this article and using the tips and tricks provided, you can master writing electron configuration in the long form.
Share Your Thoughts
What are some tips and tricks you use when writing electron configuration in the long form? Share your thoughts in the comments below.
FAQ Section
What is electron configuration?
+Electron configuration is a way of describing the arrangement of electrons in an atom.
What is the Aufbau principle?
+The Aufbau principle states that electrons fill the lowest available energy levels in an atom.
How do I write electron configuration in the long form?
+To write electron configuration in the long form, determine the number of electrons, energy levels, and orbital type, and then use the Aufbau principle to determine the number of electrons in each orbital.