Magnesium's Importance in Everyday Life

Magnesium is an essential mineral that plays a critical role in various bodily functions, including energy production, nerve function, and muscle relaxation. It is also a vital component in many industrial applications, such as the production of steel, cement, and fertilizers. One of the most fascinating aspects of magnesium is its reactivity with oxygen. In this article, we will explore three ways magnesium reacts with oxygen, highlighting the benefits, working mechanisms, and practical applications of these reactions.
Understanding Magnesium's Reactivity

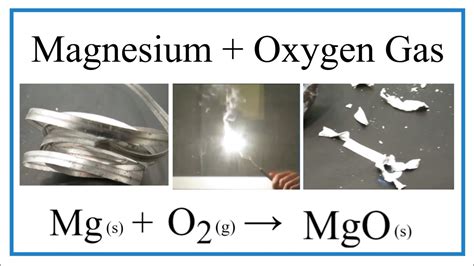
Magnesium is a highly reactive metal that readily loses two electrons to form a positive ion, known as a cation. This reactivity makes magnesium an excellent reducing agent, capable of donating electrons to other elements, including oxygen. When magnesium reacts with oxygen, it forms a compound called magnesium oxide (MgO). This reaction is highly exothermic, releasing a significant amount of heat and light energy.
Reaction 1: Combustion Reaction

One of the most spectacular ways magnesium reacts with oxygen is through a combustion reaction. When magnesium is ignited, it burns rapidly, releasing a bright white light and a significant amount of heat. This reaction is often used in fireworks and flares to produce a brilliant display of light. The reaction is highly exothermic, releasing approximately 24.9 megajoules of energy per kilogram of magnesium.
2Mg (s) + O2 (g) → 2MgO (s)
Reaction 2: Controlled Oxidation

Magnesium can also react with oxygen through a controlled oxidation reaction. In this reaction, magnesium is heated in a controlled atmosphere, allowing it to react with oxygen at a slower rate. This reaction is often used in the production of magnesium oxide, which is used as a refractory material in the steel industry.
Mg (s) + 1/2O2 (g) → MgO (s)
Reaction 3: Biological Oxidation

Magnesium also plays a critical role in biological oxidation reactions. In these reactions, magnesium acts as a cofactor, helping to facilitate the transfer of electrons in various biochemical reactions. One of the most important biological oxidation reactions involving magnesium is the production of ATP (adenosine triphosphate), which is the primary energy currency of the cell.
Benefits and Applications
The reactions between magnesium and oxygen have numerous benefits and applications. Some of the most significant advantages include:
- Energy production: Magnesium's reactivity with oxygen makes it an excellent energy source, with applications in fireworks, flares, and propulsion systems.
- Industrial applications: Magnesium oxide, produced through the reaction between magnesium and oxygen, is used as a refractory material in the steel industry and as a component in cement and fertilizers.
- Biological functions: Magnesium plays a critical role in various biological oxidation reactions, including the production of ATP, which is essential for energy production in the cell.
Practical Examples
Some practical examples of magnesium's reactivity with oxygen include:
- Fireworks: Magnesium is often used in fireworks to produce a brilliant display of light and color.
- Flares: Magnesium is used in flares to produce a bright light and heat source.
- Steel production: Magnesium oxide is used as a refractory material in the steel industry to protect against high temperatures.
Conclusion and Next Steps
In conclusion, magnesium's reactivity with oxygen is a fascinating topic with numerous benefits and applications. From energy production to industrial applications and biological functions, magnesium plays a critical role in various aspects of our lives. We hope this article has provided a comprehensive overview of the three ways magnesium reacts with oxygen and has inspired further exploration of this fascinating topic.
Now it's your turn! Share your thoughts and experiences with magnesium's reactivity in the comments section below. Have you worked with magnesium in any industrial or biological applications? Share your stories and insights with our community.
What is magnesium oxide used for?
+Magnesium oxide is used as a refractory material in the steel industry, as a component in cement and fertilizers, and in various biological applications.
What is the combustion reaction of magnesium?
+The combustion reaction of magnesium is 2Mg (s) + O2 (g) → 2MgO (s), releasing approximately 24.9 megajoules of energy per kilogram of magnesium.
What is the role of magnesium in biological oxidation reactions?
+Magnesium acts as a cofactor in biological oxidation reactions, helping to facilitate the transfer of electrons in various biochemical reactions, including the production of ATP.