Hydrochloric acid and sodium hydroxide are two of the most commonly used chemicals in various industries, including manufacturing, pharmaceuticals, and food processing. Understanding the reaction between these two substances is crucial for ensuring safe and efficient operations. In this article, we will delve into the details of the hydrochloric acid and sodium hydroxide reaction, exploring its principles, mechanisms, and practical applications.
The Importance of Understanding Chemical Reactions
Chemical reactions are the building blocks of modern industry, enabling the production of a wide range of materials, from plastics to pharmaceuticals. However, these reactions can also be hazardous if not handled properly. The reaction between hydrochloric acid and sodium hydroxide is a prime example of the importance of understanding chemical reactions. By grasping the fundamental principles of this reaction, industries can optimize their processes, minimize risks, and ensure a safer working environment.

Hydrochloric Acid and Sodium Hydroxide: An Overview
Hydrochloric acid (HCl) is a strong acid commonly used in various industrial processes, including steel production, food processing, and pharmaceutical manufacturing. Sodium hydroxide (NaOH), on the other hand, is a strong base widely used in industries such as paper production, textiles, and soap manufacturing.
The Reaction Mechanism
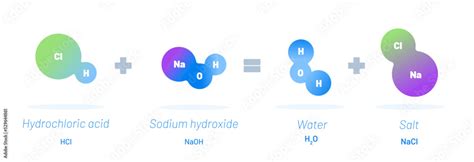
The reaction between hydrochloric acid and sodium hydroxide is a neutralization reaction, where the acid and base react to form a salt and water. The reaction is highly exothermic, releasing heat energy in the process. The balanced chemical equation for this reaction is:
HCl (aq) + NaOH (aq) → NaCl (aq) + H2O (l)
This reaction involves the transfer of a proton (H+ ion) from the hydrochloric acid molecule to the sodium hydroxide molecule, resulting in the formation of sodium chloride (NaCl) and water.
Reaction Conditions
The reaction between hydrochloric acid and sodium hydroxide is influenced by several factors, including concentration, temperature, and pressure. The reaction rate increases with increasing concentration and temperature. However, high temperatures can also lead to the decomposition of sodium hydroxide, releasing caustic fumes.

Practical Applications of the Reaction
The reaction between hydrochloric acid and sodium hydroxide has numerous practical applications across various industries. Some examples include:
- Water Treatment: The reaction is used to neutralize acidic or basic wastewater, making it safe for discharge into the environment.
- Pharmaceuticals: The reaction is used to produce various pharmaceutical products, such as antacids and pain relievers.
- Food Processing: The reaction is used to regulate the pH of food products, such as dairy products and meat.
Safety Considerations
The reaction between hydrochloric acid and sodium hydroxide can be hazardous if not handled properly. Some safety considerations include:
- Personal Protective Equipment: Wear protective clothing, gloves, and eyewear when handling hydrochloric acid and sodium hydroxide.
- Ventilation: Ensure good ventilation when handling the chemicals to prevent inhalation of caustic fumes.
- Storage: Store hydrochloric acid and sodium hydroxide in separate areas, away from incompatible materials.

Emergency Procedures
In case of an emergency, follow these procedures:
- Spill Response: Neutralize the spill with a compatible material, such as baking soda or calcium carbonate.
- Inhalation: Move to fresh air and seek medical attention if symptoms persist.
- Skin Contact: Flush the affected area with water and seek medical attention if irritation persists.
Conclusion
In conclusion, the reaction between hydrochloric acid and sodium hydroxide is a complex process that requires careful handling and attention to safety protocols. By understanding the principles and mechanisms of this reaction, industries can optimize their processes, minimize risks, and ensure a safer working environment. Remember to always follow proper safety procedures and emergency protocols when handling these chemicals.
What is the balanced chemical equation for the reaction between hydrochloric acid and sodium hydroxide?
+HCl (aq) + NaOH (aq) → NaCl (aq) + H2O (l)
What are the safety considerations when handling hydrochloric acid and sodium hydroxide?
+Wear protective clothing, gloves, and eyewear. Ensure good ventilation, and store the chemicals in separate areas, away from incompatible materials.
What are the practical applications of the reaction between hydrochloric acid and sodium hydroxide?
+The reaction is used in water treatment, pharmaceuticals, and food processing, among other industries.