Nonmetals are a class of elements that do not exhibit the typical characteristics of metals, such as luster, malleability, and conductivity. Instead, nonmetals tend to be dull, brittle, and poor conductors of electricity. Despite these differences, nonmetals are capable of forming bonds with other elements, including other nonmetals and metals. In this article, we will delve into the world of nonmetals and explore their chemical connections.
Nonmetals are located in the upper right corner of the periodic table, and they include elements such as carbon, nitrogen, oxygen, and fluorine. These elements are typically found in nature as compounds, rather than as pure elements. For example, carbon is found in the form of diamonds, graphite, and coal, while oxygen is found in the air we breathe and in water. Nonmetals can also be combined with metals to form compounds, such as rust (iron oxide) and salt (sodium chloride).
Types of Bonds Formed by Nonmetals
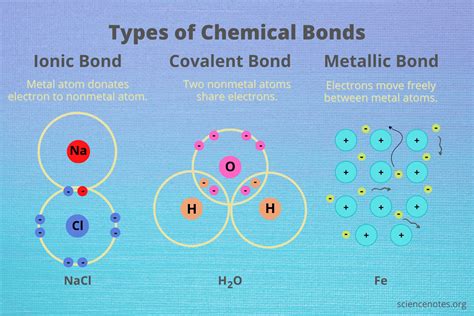
Nonmetals can form a variety of bonds with other elements, including ionic bonds, covalent bonds, and metallic bonds.

Ionic bonds are formed when a nonmetal atom gains or loses electrons to form ions with opposite charges. For example, when a sodium atom loses an electron to form a positively charged ion (Na+), it can bond with a negatively charged ion, such as chloride (Cl-), to form table salt (NaCl). Covalent bonds, on the other hand, are formed when two or more nonmetal atoms share electrons to form a molecule. For example, when two oxygen atoms share two pairs of electrons, they form a molecule of oxygen gas (O2).
Covalent Bonds: A Closer Look
Covalent bonds are the most common type of bond formed by nonmetals. In a covalent bond, the electrons are shared between the atoms, rather than being transferred from one atom to another. This type of bond is typically found in molecules, such as water (H2O) and ammonia (NH3).
Covalent bonds can be further classified into two subtypes: polar covalent bonds and nonpolar covalent bonds. Polar covalent bonds are formed when the electrons are shared unequally between the atoms, resulting in a molecule with a slight positive charge on one end and a slight negative charge on the other. Nonpolar covalent bonds, on the other hand, are formed when the electrons are shared equally between the atoms, resulting in a molecule with no net charge.
Properties of Nonmetals
Nonmetals exhibit a range of properties that distinguish them from metals. Some of the key properties of nonmetals include:

- Low melting and boiling points: Nonmetals tend to have lower melting and boiling points than metals, which means they can exist as liquids or gases at room temperature.
- Poor conductivity: Nonmetals are typically poor conductors of electricity, which means they do not allow electricity to flow through them easily.
- Dull appearance: Nonmetals tend to have a dull appearance, rather than the shiny appearance of metals.
- Brittle nature: Nonmetals are typically brittle, which means they can break or shatter easily.
Examples of Nonmetals
Some examples of nonmetals include:
- Carbon (C)
- Nitrogen (N)
- Oxygen (O)
- Fluorine (F)
- Neon (Ne)
- Chlorine (Cl)
These elements are all found in the upper right corner of the periodic table and exhibit the typical properties of nonmetals.
Applications of Nonmetals
Nonmetals have a range of applications in everyday life, including:

- Electronics: Nonmetals are used in the production of electronic components, such as semiconductors and diodes.
- Medicine: Nonmetals are used in medical treatments, such as oxygen therapy and nitrogen-rich fertilizers.
- Energy: Nonmetals are used in the production of energy, such as natural gas (mostly methane) and nuclear power.
- Materials: Nonmetals are used in the production of materials, such as plastics, ceramics, and glass.
Future Directions
Research into nonmetals is ongoing, with scientists exploring new applications and properties of these elements. Some areas of current research include:
- Nanotechnology: Scientists are exploring the properties of nonmetals at the nanoscale, which could lead to new applications in fields such as medicine and electronics.
- Energy storage: Researchers are investigating the use of nonmetals in energy storage devices, such as batteries and supercapacitors.
- Materials science: Scientists are developing new materials based on nonmetals, which could have a range of applications in fields such as aerospace and construction.
As we continue to explore the properties and applications of nonmetals, it is clear that these elements will play an increasingly important role in our daily lives.
We encourage you to share your thoughts and questions about nonmetals in the comments section below. How do you think nonmetals will be used in the future? Do you have any questions about the properties or applications of nonmetals?
What is the main difference between metals and nonmetals?
+The main difference between metals and nonmetals is their ability to conduct electricity. Metals are typically good conductors of electricity, while nonmetals are poor conductors.
What type of bond is typically formed between two nonmetal atoms?
+Covalent bonds are typically formed between two nonmetal atoms. In a covalent bond, the electrons are shared between the atoms, rather than being transferred from one atom to another.
What are some common applications of nonmetals?
+Nonmetals have a range of applications in everyday life, including electronics, medicine, energy, and materials.