Isobutyl benzoate, a chemical compound with the formula C11H14O2, is a colorless liquid with a sweet, fruity odor. It is commonly used as a fragrance ingredient and a solvent in various applications, including perfumery, cosmetics, and pharmaceuticals. One of the most common methods of synthesizing isobutyl benzoate is through the reaction of benzoic acid with isobutanol. In this article, we will explore two easy alcohol options for forming isobutyl benzoate.

Understanding Isobutyl Benzoate
Isobutyl benzoate is an ester, a type of organic compound that is formed through the reaction of an acid and an alcohol. It is a common ingredient in perfumes, cosmetics, and personal care products, where it is used to create a sweet, fruity fragrance. Isobutyl benzoate is also used as a solvent in various applications, including pharmaceuticals and food flavorings.
Importance of Isobutyl Benzoate
Isobutyl benzoate is an important compound in various industries, including:
- Perfumery: Isobutyl benzoate is used to create a sweet, fruity fragrance in perfumes and fragrances.
- Cosmetics: Isobutyl benzoate is used as a solvent and fragrance ingredient in cosmetics and personal care products.
- Pharmaceuticals: Isobutyl benzoate is used as a solvent and excipient in pharmaceutical applications.
- Food flavorings: Isobutyl benzoate is used to create a sweet, fruity flavor in food products.

Option 1: Reaction with Isobutanol
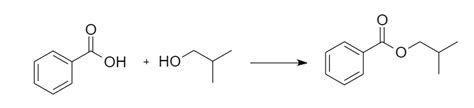
One of the most common methods of synthesizing isobutyl benzoate is through the reaction of benzoic acid with isobutanol. This reaction is a type of esterification reaction, where the acid and alcohol react to form an ester.
The reaction equation is as follows:
C6H5COOH + (CH3)2CHCH2OH → C6H5COO(CH3)2CHCH2 + H2O
In this reaction, benzoic acid reacts with isobutanol to form isobutyl benzoate and water. The reaction is typically carried out in the presence of an acid catalyst, such as sulfuric acid or hydrochloric acid.
Benefits of Using Isobutanol
Using isobutanol as the alcohol component in the synthesis of isobutyl benzoate has several benefits, including:
- High yield: The reaction of benzoic acid with isobutanol produces a high yield of isobutyl benzoate.
- Easy to handle: Isobutanol is a relatively easy compound to handle and store, making it a convenient choice for synthesis.
- Cost-effective: Isobutanol is a relatively inexpensive compound, making it a cost-effective choice for synthesis.

Option 2: Reaction with Isobutyl Chloride
Another option for forming isobutyl benzoate is through the reaction of benzoic acid with isobutyl chloride. This reaction is a type of esterification reaction, where the acid and alkyl chloride react to form an ester.
The reaction equation is as follows:
C6H5COOH + (CH3)2CHCH2Cl → C6H5COO(CH3)2CHCH2 + HCl
In this reaction, benzoic acid reacts with isobutyl chloride to form isobutyl benzoate and hydrochloric acid. The reaction is typically carried out in the presence of a base, such as sodium hydroxide or potassium hydroxide.
Benefits of Using Isobutyl Chloride
Using isobutyl chloride as the alkyl component in the synthesis of isobutyl benzoate has several benefits, including:
- High purity: The reaction of benzoic acid with isobutyl chloride produces a high-purity product.
- Easy to handle: Isobutyl chloride is a relatively easy compound to handle and store, making it a convenient choice for synthesis.
- Cost-effective: Isobutyl chloride is a relatively inexpensive compound, making it a cost-effective choice for synthesis.

Comparison of Options
Both options for forming isobutyl benzoate have their benefits and drawbacks. The reaction of benzoic acid with isobutanol produces a high yield of isobutyl benzoate, but the reaction requires an acid catalyst. The reaction of benzoic acid with isobutyl chloride produces a high-purity product, but the reaction requires a base.
In conclusion, the choice of option for forming isobutyl benzoate will depend on the specific needs and requirements of the synthesis. Both options are relatively easy to carry out and produce a high-quality product.

We hope this article has provided a helpful overview of the two easy alcohol options for forming isobutyl benzoate. If you have any questions or comments, please don't hesitate to reach out.
What is isobutyl benzoate used for?
+Isobutyl benzoate is used as a fragrance ingredient and solvent in various applications, including perfumery, cosmetics, and pharmaceuticals.
How is isobutyl benzoate synthesized?
+Isobutyl benzoate can be synthesized through the reaction of benzoic acid with isobutanol or isobutyl chloride.
What are the benefits of using isobutanol in the synthesis of isobutyl benzoate?
+The benefits of using isobutanol in the synthesis of isobutyl benzoate include high yield, easy handling, and cost-effectiveness.