The fascinating world of atomic physics! At the heart of every element lies its unique electron configuration, which determines its chemical properties and behavior. In this article, we'll delve into the intricacies of cobalt's electron configuration, exploring its significance, structure, and implications.
Cobalt, a hard, silver-white, ferromagnetic metal, is an essential component in various industries, including energy storage, catalysis, and pigmentation. Its electron configuration plays a crucial role in understanding its chemical reactivity, magnetic properties, and potential applications. Let's embark on a journey to unravel the mysteries of cobalt's electron configuration!
Understanding Electron Configuration

Before diving into cobalt's electron configuration, it's essential to grasp the basics of electron configuration itself. In an atom, electrons occupy specific energy levels or shells, which are further divided into subshells. The arrangement of electrons in these subshells determines the atom's electron configuration.
The electron configuration is typically denoted by a series of numbers and letters, indicating the energy level, subshell, and number of electrons in each subshell. For example, the electron configuration of hydrogen is 1s1, meaning one electron occupies the first energy level (1s).
Electron Configuration Notation
The notation used to describe electron configuration is based on the following rules:
- Energy levels are denoted by integers (1, 2, 3, etc.).
- Subshells are represented by letters (s, p, d, f).
- The number of electrons in each subshell is indicated by a superscript number.
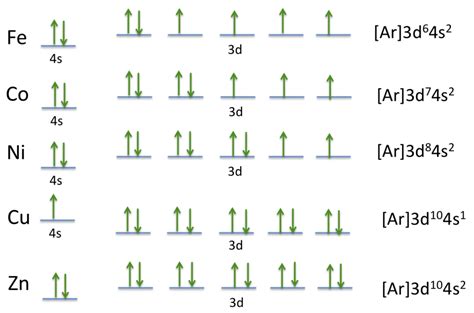
Cobalt's Electron Configuration

Cobalt's atomic number is 27, which means it has 27 protons in its atomic nucleus. To determine its electron configuration, we need to consider the Aufbau principle and the Pauli Exclusion Principle.
- The Aufbau principle states that electrons occupy the lowest available energy levels.
- The Pauli Exclusion Principle states that each subshell can hold a specific number of electrons, determined by the subshell's orbital angular momentum (l) and spin (s).
Based on these principles, cobalt's electron configuration is:
[Ar] 3d7 4s2
This notation indicates that:
- The first 18 electrons occupy the 1s, 2s, 2p, 3s, and 3p subshells ( represented by the noble gas core [Ar]).
- The next 7 electrons occupy the 3d subshell.
- The final 2 electrons occupy the 4s subshell.
Significance of Cobalt's Electron Configuration
Cobalt's electron configuration is crucial in determining its chemical properties and behavior. The partially filled 3d subshell makes cobalt a transition metal, which means it exhibits unique magnetic and catalytic properties.
- The 3d electrons are responsible for cobalt's ferromagnetic behavior, making it an essential component in magnetic materials and devices.
- The 4s electrons play a key role in cobalt's chemical reactivity, particularly in its ability to form complexes and bonds with other elements.
Applications of Cobalt's Electron Configuration

Cobalt's electron configuration has numerous practical applications:
- Energy Storage: Cobalt is a critical component in lithium-ion batteries, used in electric vehicles and portable electronics. Its electron configuration enables the efficient transfer of electrons during charging and discharging cycles.
- Catalysis: Cobalt's unique electron configuration makes it an effective catalyst in various chemical reactions, such as the production of polyethylene and polypropylene.
- Pigmentation: Cobalt's electron configuration is responsible for its vibrant blue color, making it a popular pigment in art supplies and coatings.
Challenges and Future Directions
While cobalt's electron configuration has enabled numerous applications, there are also challenges and opportunities for future research:
- Sustainability: Cobalt mining has raised environmental and social concerns. Developing more sustainable and responsible mining practices is crucial.
- Recycling: Closed-loop recycling of cobalt-containing materials can help reduce waste and conserve resources.
- New Materials: Researchers are exploring new materials with similar electron configurations to cobalt, which could lead to innovative applications and reduced dependence on cobalt.
Conclusion: Embracing the Complexity of Cobalt's Electron Configuration

In conclusion, cobalt's electron configuration is a complex and fascinating topic that underlies its unique chemical properties and applications. By understanding the intricacies of electron configuration, researchers and industry experts can develop new materials, improve existing technologies, and address the challenges associated with cobalt mining and recycling.
As we continue to explore the intricacies of atomic physics, we may uncover new secrets about cobalt's electron configuration and its potential applications. Join us in the discussion and share your thoughts on the importance of electron configuration in shaping our understanding of the world around us!
FAQ Section
What is electron configuration?
+Electron configuration is the arrangement of electrons in an atom, which determines its chemical properties and behavior.
What is the electron configuration of cobalt?
+Cobalt's electron configuration is [Ar] 3d7 4s2.
What are the applications of cobalt's electron configuration?
+Cobalt's electron configuration has numerous applications, including energy storage, catalysis, and pigmentation.