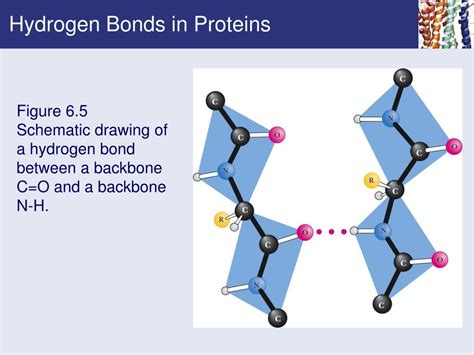
Serine is a polar amino acid that plays a crucial role in the structure and function of proteins. One of the key properties of serine is its ability to form hydrogen bonds, which are essential for maintaining the stability and function of proteins.
Hydrogen bonds are weak electrostatic attractions between atoms that have a partial positive charge and atoms that have a partial negative charge. In proteins, hydrogen bonds are formed between the backbone nitrogen and oxygen atoms, as well as between side chains of polar amino acids, such as serine.

Serine's side chain, which contains a hydroxyl (-OH) group, is capable of forming hydrogen bonds with other polar atoms in the protein. This is particularly important in the context of protein structure and function, as hydrogen bonds help to stabilize the protein's native conformation and facilitate interactions with other molecules.
How Does Serine Form Hydrogen Bonds in Proteins?
Serine's ability to form hydrogen bonds is due to the presence of the hydroxyl group in its side chain. The hydroxyl group is polar, meaning it has a partial positive charge on the hydrogen atom and a partial negative charge on the oxygen atom. This polarity allows serine to form hydrogen bonds with other polar atoms in the protein.
In particular, serine's hydroxyl group can form hydrogen bonds with the backbone nitrogen and oxygen atoms of other amino acids, as well as with the side chains of other polar amino acids. These hydrogen bonds help to stabilize the protein's native conformation and facilitate interactions with other molecules.
The Importance of Serine's Hydrogen Bonding Ability
Serine's ability to form hydrogen bonds is essential for the structure and function of many proteins. For example, serine is often found in the active sites of enzymes, where it plays a crucial role in catalyzing chemical reactions. The hydrogen bonding ability of serine helps to position the enzyme's substrates and facilitate the chemical reaction.
In addition, serine's hydrogen bonding ability is also important for protein-protein interactions. Many proteins contain serine-rich regions that are involved in interactions with other proteins or molecules. The hydrogen bonding ability of serine helps to stabilize these interactions and facilitate the formation of protein complexes.

Examples of Serine's Role in Hydrogen Bonding
There are many examples of serine's role in hydrogen bonding in proteins. One well-known example is the enzyme ribonuclease A, which contains several serine residues in its active site. The hydroxyl groups of these serine residues form hydrogen bonds with the substrate, helping to position it for catalysis.
Another example is the protein lysozyme, which contains a serine-rich region that is involved in interactions with the bacterial cell wall. The hydrogen bonding ability of serine helps to stabilize these interactions and facilitate the binding of lysozyme to the bacterial cell wall.
Conclusion
In conclusion, serine's ability to form hydrogen bonds is essential for the structure and function of many proteins. The polar nature of serine's side chain allows it to form hydrogen bonds with other polar atoms in the protein, helping to stabilize the protein's native conformation and facilitate interactions with other molecules. The importance of serine's hydrogen bonding ability is demonstrated by its role in many enzymes and protein-protein interactions.
We hope this article has provided you with a comprehensive understanding of serine's role in hydrogen bonding in proteins. If you have any questions or would like to share your thoughts, please leave a comment below.
What is the role of serine in protein structure and function?
+Serine plays a crucial role in protein structure and function, particularly in the context of hydrogen bonding. Its polar side chain allows it to form hydrogen bonds with other polar atoms in the protein, helping to stabilize the protein's native conformation and facilitate interactions with other molecules.
How does serine form hydrogen bonds in proteins?
+Serine's hydroxyl group is polar, meaning it has a partial positive charge on the hydrogen atom and a partial negative charge on the oxygen atom. This polarity allows serine to form hydrogen bonds with other polar atoms in the protein, such as the backbone nitrogen and oxygen atoms, as well as with the side chains of other polar amino acids.
What are some examples of serine's role in hydrogen bonding in proteins?
+There are many examples of serine's role in hydrogen bonding in proteins, including its role in the enzyme ribonuclease A and the protein lysozyme. In these proteins, serine's hydrogen bonding ability helps to stabilize the protein's native conformation and facilitate interactions with other molecules.