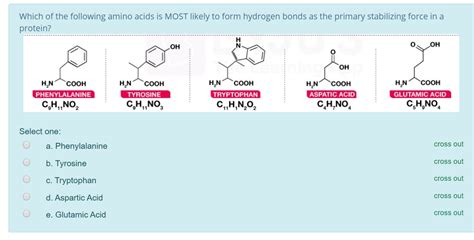
Phenylalanine is an essential amino acid that plays a crucial role in various biological processes. One of its key properties is its ability to form hydrogen bonds, which are weak electrostatic attractions between atoms with a partial positive charge and atoms with a partial negative charge.
Phenylalanine's structure consists of a non-polar, hydrophobic side chain and a polar, hydrophilic amino group. The amino group is capable of forming hydrogen bonds with other molecules, which is essential for its biological functions.

Hydrogen Bonding in Phenylalanine
Hydrogen bonds are relatively weak compared to covalent bonds, but they play a crucial role in the structure and function of biomolecules. Phenylalanine's amino group can form hydrogen bonds with other molecules through its nitrogen atom, which has a partial positive charge.
The hydrogen bonding capabilities of phenylalanine are influenced by its side chain. The non-polar, hydrophobic nature of the side chain makes it less likely to participate in hydrogen bonding. However, the amino group is still capable of forming hydrogen bonds, which is essential for its biological functions.
Importance of Hydrogen Bonding in Phenylalanine
Hydrogen bonding is essential for phenylalanine's biological functions. It plays a crucial role in protein structure and function, as well as in enzyme-substrate interactions.
In proteins, hydrogen bonds between phenylalanine residues and other amino acids help to stabilize the protein structure. These bonds also contribute to the protein's functional properties, such as its ability to bind to specific molecules or to catalyze chemical reactions.
Factors Affecting Hydrogen Bonding in Phenylalanine
Several factors can affect phenylalanine's ability to form hydrogen bonds. These include:
- pH: Changes in pH can affect the ionization state of the amino group, which can influence its ability to form hydrogen bonds.
- Temperature: Increasing temperature can reduce the strength of hydrogen bonds, while decreasing temperature can increase their strength.
- Solvent: The solvent in which phenylalanine is dissolved can affect its ability to form hydrogen bonds. Polar solvents, such as water, can enhance hydrogen bonding, while non-polar solvents can reduce it.
Practical Applications of Phenylalanine's Hydrogen Bonding Properties
The hydrogen bonding properties of phenylalanine have several practical applications. These include:
- Protein engineering: Understanding the hydrogen bonding properties of phenylalanine can help researchers design proteins with specific functional properties.
- Drug design: The ability of phenylalanine to form hydrogen bonds can be exploited in the design of drugs that target specific proteins or enzymes.
- Biotechnology: Phenylalanine's hydrogen bonding properties can be used to develop biotechnology applications, such as biosensors and biochips.

Conclusion
Phenylalanine's ability to form hydrogen bonds is a crucial aspect of its biological functions. Understanding the factors that affect its hydrogen bonding properties can help researchers design proteins and drugs with specific functional properties. The practical applications of phenylalanine's hydrogen bonding properties are diverse and continue to grow as research in this area advances.
Stay Engaged
We hope this article has provided valuable insights into the hydrogen bonding properties of phenylalanine. If you have any questions or comments, please feel free to share them below. Don't forget to share this article with your colleagues and friends who may be interested in learning more about phenylalanine's hydrogen bonding properties.
What is the role of hydrogen bonding in phenylalanine's biological functions?
+Hydrogen bonding plays a crucial role in phenylalanine's biological functions, including protein structure and function, as well as enzyme-substrate interactions.
What factors can affect phenylalanine's ability to form hydrogen bonds?
+Several factors can affect phenylalanine's ability to form hydrogen bonds, including pH, temperature, and solvent.
What are some practical applications of phenylalanine's hydrogen bonding properties?
+The practical applications of phenylalanine's hydrogen bonding properties include protein engineering, drug design, and biotechnology.