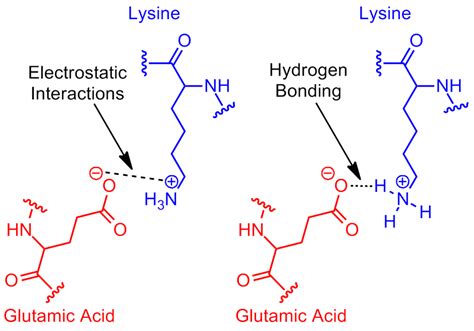
In the realm of biochemistry, amino acids play a vital role in the formation of proteins, which are the building blocks of life. One such amino acid, lysine, is particularly fascinating due to its unique properties and functions. Lysine is an essential amino acid, meaning it cannot be produced by the human body and must be obtained through diet. Its role in the human body is multifaceted, and one of the key aspects of its functionality is its ability to form hydrogen bonds. In this article, we will delve into the three ways lysine forms hydrogen bonds, exploring the intricacies of this process and its significance in biological systems.
Lysine Structure and Properties

Before we dive into the specifics of hydrogen bonding, it's essential to understand the structure and properties of lysine. Lysine is a positively charged amino acid, also known as a basic amino acid. Its chemical formula is C6H14N2O2, and it contains an amino group (-NH2) and a carboxyl group (-COOH). The unique aspect of lysine is its long side chain, which is positively charged at physiological pH. This property allows lysine to interact with other molecules and form various types of bonds.
Hydrogen Bonding: A Crucial Aspect of Lysine's Functionality
Hydrogen bonding is a type of intermolecular force that arises between atoms with a partial positive charge (hydrogen atoms bonded to highly electronegative atoms) and atoms with a partial negative charge. In the context of lysine, hydrogen bonding plays a vital role in its interactions with other molecules. The three ways lysine forms hydrogen bonds are:
1. N-H...O Hydrogen Bonding
This type of hydrogen bonding occurs between the nitrogen atom of lysine's amino group and the oxygen atom of a nearby molecule. The positively charged nitrogen atom forms a hydrogen bond with the negatively charged oxygen atom, resulting in a weak electrostatic attraction. This type of bonding is essential for the formation of protein structures, such as alpha helices and beta sheets.
2. N-H...N Hydrogen Bonding
In this type of hydrogen bonding, the nitrogen atom of lysine's amino group forms a bond with the nitrogen atom of another molecule. This type of bonding is less common than N-H...O hydrogen bonding but is still crucial for the stability of protein structures.
3. N-H...π Hydrogen Bonding
This type of hydrogen bonding occurs between the nitrogen atom of lysine's amino group and the π electrons of an aromatic ring. The positively charged nitrogen atom forms a weak electrostatic attraction with the negatively charged π electrons, resulting in a stabilizing interaction.
Importance of Lysine's Hydrogen Bonding in Biological Systems

The ability of lysine to form hydrogen bonds is crucial for various biological processes. Some of the key importance of lysine's hydrogen bonding include:
- Protein structure and stability: Hydrogen bonding helps to stabilize the structure of proteins, allowing them to perform their biological functions.
- Enzyme-substrate interactions: Hydrogen bonding plays a crucial role in the interaction between enzymes and their substrates, facilitating the catalysis of biochemical reactions.
- Cell signaling: Hydrogen bonding is involved in the transmission of signals between cells, allowing for communication and coordination of cellular activities.
Practical Applications of Lysine's Hydrogen Bonding
The understanding of lysine's hydrogen bonding has various practical applications in fields such as:
- Protein engineering: Knowledge of lysine's hydrogen bonding can be used to design proteins with specific functions and properties.
- Drug development: Understanding the role of lysine's hydrogen bonding in enzyme-substrate interactions can aid in the development of targeted drugs.
- Food science: Lysine's hydrogen bonding can be used to improve the texture and stability of food products.
What is the chemical formula of lysine?
+The chemical formula of lysine is C6H14N2O2.
What is the significance of lysine's long side chain?
+Lysine's long side chain is positively charged at physiological pH, allowing it to interact with other molecules and form various types of bonds.
What are the three ways lysine forms hydrogen bonds?
+Lysine forms hydrogen bonds through N-H...O, N-H...N, and N-H...π interactions.
In conclusion, the ability of lysine to form hydrogen bonds is a vital aspect of its functionality in biological systems. Understanding the three ways lysine forms hydrogen bonds and their significance in protein structure, enzyme-substrate interactions, and cell signaling can provide valuable insights into the complex world of biochemistry. As we continue to explore the intricacies of lysine's hydrogen bonding, we may uncover new practical applications in fields such as protein engineering, drug development, and food science.
We invite you to share your thoughts on the importance of lysine's hydrogen bonding in the comments section below. What are some potential applications of lysine's hydrogen bonding that you think are worth exploring?