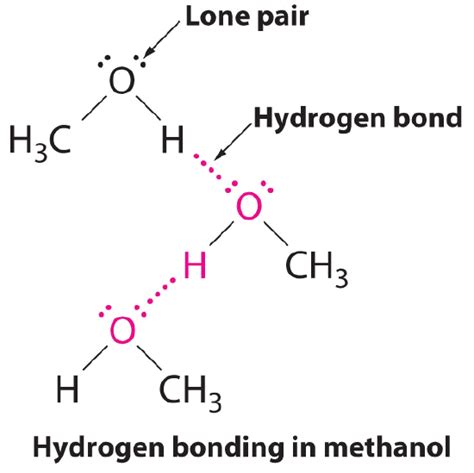
Hydrogen bonding is a crucial aspect of chemistry, particularly in understanding the behavior of molecules in various environments. Methanol, a simple alcohol with the chemical formula CH₃OH, exhibits unique properties due to its ability to form hydrogen bonds. This article will delve into the three primary ways methanol forms hydrogen bonds, exploring the underlying mechanisms and the implications of these interactions.
Methanol's Structure and Hydrogen Bonding

Methanol's molecular structure consists of a methyl group (CH₃) attached to a hydroxyl group (OH). The presence of the hydroxyl group, which contains a hydrogen atom bonded to an oxygen atom, is crucial for hydrogen bonding. Oxygen is a highly electronegative atom, drawing electrons away from the hydrogen atom and creating a partial positive charge on the hydrogen. This partial positive charge allows the hydrogen atom to interact with electronegative atoms in other molecules, forming hydrogen bonds.
1. Methanol-Methanol Hydrogen Bonding
Intermolecular Hydrogen Bonding
Methanol molecules can form hydrogen bonds with each other through intermolecular interactions. In this scenario, the hydrogen atom of one methanol molecule is attracted to the oxygen atom of another methanol molecule. This attraction is due to the partial positive charge on the hydrogen atom and the partial negative charge on the oxygen atom.
Hydrogen Bonding in Methanol Dimers
Studies have shown that methanol molecules can form dimers, where two methanol molecules are connected through hydrogen bonds. These dimers are stabilized by the attractive forces between the hydrogen atoms and oxygen atoms of adjacent molecules. The formation of methanol dimers is an important aspect of its physical and chemical properties, influencing its boiling point, solubility, and reactivity.
2. Methanol-Water Hydrogen Bonding
Hydrogen Bonding with Water Molecules
Methanol can also form hydrogen bonds with water molecules. In this case, the hydrogen atom of methanol is attracted to the oxygen atom of a water molecule. This interaction is essential for understanding the solubility of methanol in water. The ability of methanol to form hydrogen bonds with water molecules allows it to dissolve in water, making it an important solvent in various applications.
Implications of Methanol-Water Hydrogen Bonding
The hydrogen bonding between methanol and water molecules has significant implications for its use in biological systems. For example, methanol is used as a solvent in some biochemical reactions, where its ability to form hydrogen bonds with water molecules helps to facilitate the reaction.
3. Methanol-Other Molecules Hydrogen Bonding
Hydrogen Bonding with Other Molecules
Methanol can form hydrogen bonds with a variety of other molecules, including those containing electronegative atoms such as nitrogen, fluorine, and chlorine. These interactions are essential for understanding the physical and chemical properties of methanol-based systems.
Examples of Methanol-Other Molecules Hydrogen Bonding
One example of methanol's hydrogen bonding with other molecules is its interaction with ammonia (NH₃). In this case, the hydrogen atom of methanol is attracted to the nitrogen atom of ammonia, forming a hydrogen bond. This interaction is important for understanding the behavior of methanol in various applications, including its use as a solvent in chemical reactions.
Conclusion
In conclusion, methanol forms hydrogen bonds through three primary mechanisms: methanol-methanol, methanol-water, and methanol-other molecules. These interactions are crucial for understanding the physical and chemical properties of methanol, including its solubility, boiling point, and reactivity. By recognizing the importance of hydrogen bonding in methanol-based systems, we can better design and optimize various applications, from biochemical reactions to solvent-based processes.
Call to Action
We hope this article has provided a comprehensive overview of methanol's hydrogen bonding mechanisms. If you have any questions or would like to learn more about this topic, please don't hesitate to comment below. Share this article with your colleagues and friends to spread the knowledge!
FAQ Section
What is the chemical structure of methanol?
+Methanol's chemical structure consists of a methyl group (CH₃) attached to a hydroxyl group (OH).
How does methanol form hydrogen bonds with other molecules?
+Methanol forms hydrogen bonds with other molecules through the attraction between its hydrogen atom and the electronegative atoms of other molecules.
What are the implications of methanol's hydrogen bonding with water molecules?
+The hydrogen bonding between methanol and water molecules has significant implications for its use in biological systems, including its use as a solvent in biochemical reactions.