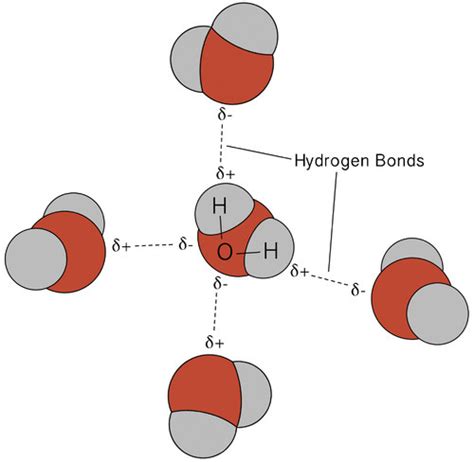
At the core of every living organism, from the simplest bacteria to the most complex forms of life, lies a fundamental building block: the molecule. And at the heart of many of these molecules is a simple, yet extraordinary, substance: hydrogen. Formed from just two atoms, hydrogen is the lightest and most abundant chemical element in the universe. Its unique properties make it an essential component of countless biological and chemical processes, and its role in the formation of molecules is a fascinating story that has captivated scientists for centuries.
In this article, we will delve into the world of hydrogen, exploring its properties, its role in the formation of molecules, and its importance in various biological and chemical processes. We will also examine the fascinating ways in which hydrogen atoms interact with each other and with other elements to form the complex structures that underlie all of life.

What is Hydrogen?
Hydrogen is the lightest and most abundant chemical element in the universe, making up approximately 75% of its elemental mass. It is a colorless, odorless, and highly flammable gas that is found naturally in many forms, including water, hydrocarbons, and other organic compounds. Hydrogen has several unique properties that make it an essential component of many biological and chemical processes. It has a high energy density, making it an excellent fuel source, and its high reactivity allows it to form bonds with many other elements.
Properties of Hydrogen
Some of the key properties of hydrogen include:
- High energy density: Hydrogen has a high energy density, making it an excellent fuel source.
- High reactivity: Hydrogen is highly reactive, allowing it to form bonds with many other elements.
- Low atomic mass: Hydrogen has the lowest atomic mass of all elements, making it highly mobile and able to penetrate many materials.
- High thermal conductivity: Hydrogen has a high thermal conductivity, making it an excellent heat transfer medium.

How Do Atoms Form Hydrogen?
Hydrogen is formed when two hydrogen atoms bond together to form a molecule. This process is known as atomic bonding, and it is the fundamental mechanism by which all molecules are formed. The bonding process involves the sharing of electrons between the two atoms, which creates a strong attractive force between them.
The Atomic Bonding Process
The atomic bonding process involves several key steps:
- Atomic attraction: The two hydrogen atoms are attracted to each other due to the electrostatic force between their positively charged nuclei and negatively charged electrons.
- Electron sharing: The electrons in the outermost energy level of each atom are shared between the two atoms, creating a strong attractive force between them.
- Bond formation: The shared electrons create a strong bond between the two atoms, holding them together in a molecule.

Importance of Hydrogen in Biological Processes
Hydrogen plays a crucial role in many biological processes, including:
- Energy production: Hydrogen is a key component of the energy-producing molecule ATP (adenosine triphosphate).
- Metabolism: Hydrogen is involved in many metabolic processes, including the breakdown of glucose and the synthesis of fatty acids.
- DNA synthesis: Hydrogen is a key component of the nucleotides that make up DNA.
Examples of Hydrogen in Biological Processes
Some examples of hydrogen in biological processes include:
- The citric acid cycle: Hydrogen is involved in the breakdown of glucose in the citric acid cycle.
- Fatty acid synthesis: Hydrogen is involved in the synthesis of fatty acids from acetyl-CoA.
- DNA replication: Hydrogen is involved in the synthesis of nucleotides during DNA replication.

Importance of Hydrogen in Chemical Processes
Hydrogen is also an essential component of many chemical processes, including:
- Industrial synthesis: Hydrogen is used as a reactant in the synthesis of many industrial chemicals, including ammonia and methanol.
- Fuel production: Hydrogen is used as a fuel source in the production of electricity and transportation fuels.
- Chemical reactions: Hydrogen is involved in many chemical reactions, including the Haber-Bosch process for the production of ammonia.
Examples of Hydrogen in Chemical Processes
Some examples of hydrogen in chemical processes include:
- The Haber-Bosch process: Hydrogen is involved in the production of ammonia from nitrogen and hydrogen.
- Methanol synthesis: Hydrogen is used as a reactant in the synthesis of methanol from carbon dioxide and hydrogen.
- Fuel cell reactions: Hydrogen is involved in the reaction of fuel cells to produce electricity.

What is the atomic mass of hydrogen?
+The atomic mass of hydrogen is 1.00794 u (unified atomic mass units).
What is the most common isotope of hydrogen?
+The most common isotope of hydrogen is protium, which has one proton and no neutrons in its nucleus.
What is the role of hydrogen in the formation of molecules?
+Hydrogen plays a crucial role in the formation of molecules, as it is able to form bonds with many other elements, including oxygen, carbon, and nitrogen.
We hope this article has provided you with a deeper understanding of the fascinating world of hydrogen and its role in the formation of molecules. Whether you are a scientist, a student, or simply someone with a curiosity about the natural world, we encourage you to continue exploring the many wonders of hydrogen and its importance in our universe.